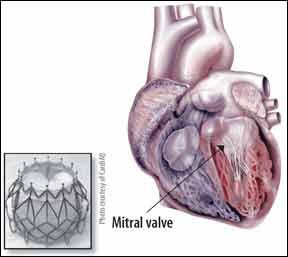
Of the estimated 3.7 million Americans with severe mitral regurgitation (MR) and heart failure (HF), as many as 2.5 million have no real treatment options. Thats because theyre too sick or frail to handle open heart surgery to replace their ailing valves. But the options for inoperable and high-risk surgical candidates are changing after the first successful implantation of a transcatheter prosthetic mitral valve in a human patient earlier this year. Doctors in Denmark implanted the CardiAQ TMVI bioprosthetic valve in an 86-year-old man with severe mitral regurgitation. The procedure is performed by guiding the prosthetic valve at the end of a catheter up through a blood vessel to the location of the defective mitral valve. The CardiAQ valve then self-expands into place over the patients own valve and is secured in place by a mechanism that takes advantage of the foreshortening of the frame during diagonal expansion.
To continue reading this article or issue you must be a paid subscriber.
Sign in