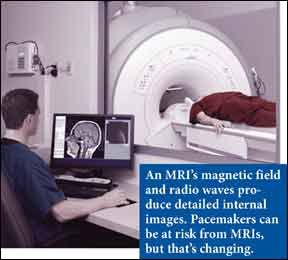
Nearly two million Americans have pacemakers, devices that use electrical charges to help keep the heart beating in a synchronized rhythm, and many of those heart patients will develop cancer or another medical conditions that requires a magnetic resonance imaging exam (MRI). However, the strong magnetic fields of MRI can disrupt the pacemakers functioning and cause pacemaker wires to overheat. But the U.S. Food and Drug Administration recently approved the first MRI-safe pacemaker. Medtronics REVO MRI pacemaker system is approved for rate-adaptive pacing and dual-chamber pacing.
To continue reading this article or issue you must be a paid subscriber.
Sign in